Previously on this blog I've written about Copaxone and its "mechanism of the week". For example, here and here. I haven't done it for a while, but can't resist bringing this one to your attention.
Now it's anti-bacterial. What next? It inhibits EBV :-)
But I guess it must be working by blocking the microbiome:-).
Christiansen SH, Murphy RA, Juul-Madsen K, Fredborg M, Hvam ML, Axelgaard E, Skovdal SM, Meyer RL, Sørensen UBS, Möller A, Nyengaard JR, Nørskov-Lauritsen N, Wang M, Gadjeva M, Howard KA, Davies JC, Petersen E, Vorup-Jensen T. The Immunomodulatory Drug Glatiramer Acetate is Also an Effective Antimicrobial Agent that Kills Gram-negative Bacteria.
Sci Rep. 2017; 7(1):15653. doi: 10.1038/s41598-017-15969-3.
Classic drug development strategies have failed to meet the urgent clinical needs in treating infections with Gram-negative bacteria. Repurposing drugs can lead to timely availability of new antibiotics, accelerated by existing safety profiles. Glatiramer acetate (GA) is a widely used and safe formulation for treatment of multiple sclerosis. It contains a large diversity of essentially isomeric polypeptides with the cationic and amphiphilic character of many antimicrobial peptides (AMP). Here, we report that GA is antibacterial, targeting Gram-negative organisms with higher activity towards Pseudomonas aeruginosa than the naturally-occurring AMP LL-37 in human plasma. As judged from flow cytometric assays, bacterial killing by GA occurred within minutes. Laboratory strains of Escherichia coli and P. aeruginosa were killed by a process of condensing intracellular contents. Efficient killing by GA was also demonstrated in Acinetobacter baumannii clinical isolates and approximately 50% of clinical isolates of P. aeruginosa from chronic airway infection in CF patients. By contrast, the Gram-positive Staphylococcus aureus cells appeared to be protected from GA by an increased formation of nm-scale particulates. Our data identify GA as an attractive drug repurposing candidate to treat infections with Gram-negative bacteria.
And whilst on the subject:
Grossman I, Kolitz S, Komlosh A, Zeskind B, Weinstein V, Laifenfeld D, Gilbert A, Bar-Ilan O, Fowler KD, Hasson T, Konya A, Wells-Knecht K, Loupe P, Melamed-Gal S, Molotsky T, Krispin R, Papir G, Sahly Y, Hayden MR. Compositional differences between Copaxone and Glatopa are reflected in altered immunomodulation ex vivo in a mouse model. Ann N Y Acad Sci. 2017 1407(1):75-89.
Glatiramer acetate is big business as it is the number one selling drug. But there's one problem: (no, not its level of efficacy) Copaxone is now out of patent protection and generics are entering the market place.
So what do you do to protect your product?
You aim to show that the competitor compound is different?
Copaxone is a random mix of 4 amino acids and so different batches of the drug are different.
Glatopa (a generic Copaxone that induces some genes different to Copaxone) is one variant, but is it therapeutically different?
So what do you do to protect your product?
You aim to show that the competitor compound is different?
Copaxone is a random mix of 4 amino acids and so different batches of the drug are different.
Glatopa (a generic Copaxone that induces some genes different to Copaxone) is one variant, but is it therapeutically different?







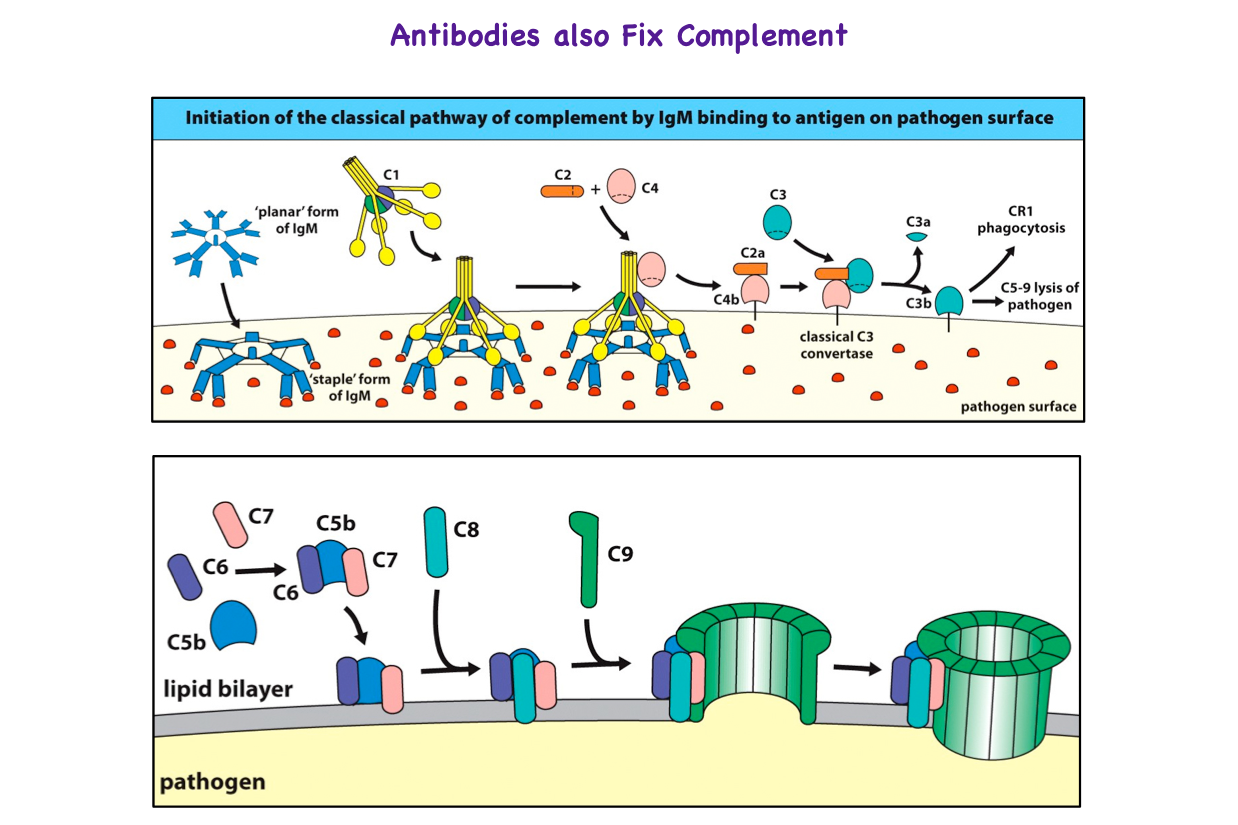
.png/800px-Membrane_Attack_Complex_(Terminal_Complement_Complex_C5b-9).png)